
|
Animal
Models of Psychiatric Disorders
Author
Paul
Kenyon
|
|
Measuring animal
behaviour under laboratory conditions - the Skinner box
An area of
psychology called psychopharmacology
or behavioural pharmacology is concerned
with measuring the effects of drugs on
behaviour. Exploring the effects of drugs on operant schedules of
reinforcement has a long
tradition in psychopharmacology. Here is some of the technology used by
psychopharmacologists.
 This picture gives an
idea of the facilities available in an operant chamber, or Skinner box.
This picture gives an
idea of the facilities available in an operant chamber, or Skinner box.
The box
is equipped with a lever; responses
on the lever are recorded on a cumulative recorder (see below).
Reinforcements in the form
of food, water or electric shock can be delivered according to the
contingencies of
reinforcement set-up by the experimenter.
Electric
shock can be delivered to the
animal's feet through the floor of the box which consists of a grid of
parallel metal
rods. Food or water can be delivered through a food trough beside the
lever.
The box
is equipped with lights and a speaker
for the delivery of stimuli which can come to control the rat's
behaviour.
In many
studies the animal's bar presses and
the delivery of stimuli are recorded on a cumulative
recorder.
Cumulative
recorder: Diagrammatic representation

How a cumulative recorder works . The
paper unrolls ( yellow
roller ) under the two pens at a constant speed
(normally 5mm/min, but speeded up in this demonstration).
This animation shows the
process:
- Run the animation
- Stop the animation
Each
bar-press moves the response
marking pen a small increment to the left along a brown
steel bar . Note that when the pen reaches the
left hand edge of the paper, it
is automatically reset to the baseline on the right hand side. An automatic
reset is
indicated by a vertical line running from left to right of the paper.
Automatic resets can
be programmed to occur at fixed time intervals e.g. every 15 minutes .
Reinforcements are indicated by a short blue diagonal
slash on the cumulative record.
 The relative angles of
successive slopes gives a
visual indication of the regularity in response rate. Steep slopes
reflect high response
rates and vice versa.
The relative angles of
successive slopes gives a
visual indication of the regularity in response rate. Steep slopes
reflect high response
rates and vice versa.
Rationale
for animal models of human behaviour
 |
A model
is a simple representation of a complex system. For example, a model
aeroplane or train looks similar to the real thing but lacks all the
features of the full size object. An animal model of a psychiatric
disorder is an attempt to capture the essence of the condition, but it
does not claim to reproduce the human condition in an animal.
Depression, schizophrenia and anxiety are probably uniquely human
conditions. One purpose of an animal model is to discover novel
medicines that combat the abnormal behaviour in the animal model which
could be then be used to alleviate human suffering. |
 |
The
development of an animal model often
begins with the accidental discovery that a drug produces abnormal
behaviour in otherwise
normal people. Psychologists interested in the biological bases of
abnormal behaviour then
follow these five steps to determine whether it would be worthwhile
using the drug to
construct an animal model of the abnormal human behaviour.
| Steps in the
construction of an animal model |
| 1
|
Drug
X has a behavioural effect in humans that resembles human pathology
e.g. drug X produces depression when given to humans |
| 2
|
Drug
X produces similar behavioural effects in animals, allowing for any
species differences in behaviour |
| 3
|
Drug
X produces specific biochemical effects in animals |
| 4
|
Thus
the biochemical effects produced in animals provide data relevant to
the behavioural effect of drug X in humans |
| 5
|
If
the behavioural effect of drug X in humans has the same characteristics
as pathological behaviour, then the biochemical changes produced by
drug X in animals may also provide data relevant for the understanding
of the abnormal human behaviour. |
Depression:The
catecholamine theory of mood
The
catecholamine theory of mood suggests
that:
- Depression
is associated with a catecholamine deficiency in the
brain
- Mania
is associated with a catecholamine excess in the brain
The
catecholamine-depleting drugs
tetrabenazine and reserpine have been explored for their ability to
produce animal models
of depression. Here are some stills from an animation showing the
effects of these drugs
on catecholamines.
You can
download the movie - which has
audio effects and larger images.
One of
the first animal models of depression
involved reducing catecholamine levels in the brain. Here are the steps
that encouraged
neuroscientists to develop an animal model based on injecting rats with
reserpine or
tetrabenazine.
| Rationale for the
tetrabenazine/reserpine animal model of depression |
| 1
|
About
15% of humans treated with reserpine for hypertension develop
depression |
| 2
|
Reserpine
and TBZ disrupt conditioned and unconditioned behaviour in animals |
| 3
|
Reserpine
and TBZ produce profound depletion of catecholamines |
| 4
|
Thus
the depletion in CAs may be related to human depression - this is the
CA theory of Mood |
| 5
|
The
depression produced by reserpine in humans is similar to 'naturally
occurring' depression. Thus CA depletion may be responsible for
'naturally occurring' depression. |
Sidman
avoidance schedule
In order to
explore this model of depression,
rats that had been trained on an avoidance schedule in a Skinner box
were injected with
reserpine or tetrabenazine. Avoidance schedules of reinforcement are
very useful to
psychopharmacologists because the rat's motivation remains relatively
stable during a test
session. In contrast, on schedules of reinforcement maintained by food
rewards the rat may
grow less hungry during a long test session because it is receiving
food pellets for
pressing the lever. In contrast, the motivation to avoid shock does not
wane with the
passage of time.
A rat
working on a Sidman avoidance schedule
has no external signal to mark the onset of shock. A shock is delivered
to the rat that
lasts 1-2 seconds at regularly spaced intervals (the shock-shock
interval ;
normally 5 seconds). The presentation of shock can be delayed by a
fixed time interval (
the response-shock interval, normally 20
seconds) if the rat presses a
lever.
 For example, the library
has us all
on a Sidman avoidance schedule. You can borrow a book for 14 days. If
you take it back
before the loan period is up, you can sign it out for another 14 days.
If you don't take
it back within 14 days you start to get fined. Every day you keep the
book beyond the loan
period the fine rises. Thus the library is operating a 14 day response-shock
(borrow-return)
interval, and a 1 day shock-shock (fine-fine)
interval. A smart person who was a
slow reader would return the book to the library every 13-14 days and
keep renewing the
loan period.
For example, the library
has us all
on a Sidman avoidance schedule. You can borrow a book for 14 days. If
you take it back
before the loan period is up, you can sign it out for another 14 days.
If you don't take
it back within 14 days you start to get fined. Every day you keep the
book beyond the loan
period the fine rises. Thus the library is operating a 14 day response-shock
(borrow-return)
interval, and a 1 day shock-shock (fine-fine)
interval. A smart person who was a
slow reader would return the book to the library every 13-14 days and
keep renewing the
loan period.
Well
trained rats can show very accurate time
discriminations - responding every 19 seconds and rarely getting
shocked; we don't really
know how they do this, but they certainly don't wear Rolex!
Tetrabenazine: An
animal model of depression?
Tetrabenazine
(TBZ) depletes catecholamines (NA
& DA) and was used to test the Catecholamine Theory of Mood.
The picture below is a
cumulative record showing the effects of tetrabenazine on
the avoidance behaviour of a rat trained to avoid electric shock on a
Sidman avoidance schedule. Compare control ( A-1,A-2
) performance (steady response rate) with the effects
of 2.0 mg/kg TBZ (B1-B3 ).
Response rate declines dramatically about 25 min after injection of the
drug. |
 |
 |
The next set of
cumulative records shows the protective effects of the antidepressant
drug iproniazid - a monoamine oxidase inhibitor
(MAOI) - against tetrabenazine.
Note how response rate is actually increased following
pre-treatment with iproniazid and tetrabenazine. This effect is
outlined in red on the diagram. |
 |
Tetrabenazine and
imipramine
At this
point in the story you might think we
have succeeded in producing a pretty good animal model of human
depression that could be
used to test novel drugs for antidepressant potential. The logic would
be that if a new
drug reversed the disruption of conditioned avoidance behaviour
produced by 2.0 mg/kg TBZ
then it would be a potential candidate for clinical trials in humans.
But there is a sting
in the tail of this story.
The
first thing we need to do is ask whether
other antidepressant drugs reverse TBZ-induced behavioural disruption
in our rat model. We
already know that tricyclic drugs such as imipramine are effective
antidepressants.
Unfortunately imipramine does not antagonize the effects of 2.0
mg/kg TBZ.
But
imipramine does interact with TBZ in an interesting way. A very low
dose of TBZ (0.2 mg/kg) on its own does not
interfere with conditioned avoidance behaviour. When this low dose of
TBZ is given in combination with imipramine, a period of behavioural excitation is
seen - but not until 2.25 hours after the TBZ injection.
This effect is illustrated in the next diagram. The late period of
excitation is outlined in red |
 |
Summary of TBZ
antagonism studies
Here is a
summary of the current state of our
slightly battered animal model of human depression.
| |
Antagonism of
tetrabenazine at |
| Antidepressant
group |
0.2
mg/kg |
2.0
mg/kg |
| Tricyclics
|
Yes |
No |
| MAOIs
|
No |
Yes |
One
further problem with this model is the
issue of theraputic-lag. It been known for some
time that the antidepressant
effects of drugs like imipramine take some time to develop - typically
21 days or more.
This is clearly at variance with the rapid changes of behaviour seen in
the animal model
we examined.
You can
read a fuller account of The Catecholamine
Theory of Mood
Learned
helplessness theory of depression

Martin
Seligman is responsible for learned
helplessness theory which had a major influence on psychological
research into depression
in the 1970s. Seligman discovered helplessness by accident whilst
studying the effects of
inescapable shock on active avoidance learning in dogs.
Seligman
restrained dogs in a Pavlovian
harness and administered several shocks (UCS) paired with a conditioned
stimulus (CS) -
this is the conventional CS-UCS pairing procedure used to study classical
conditioning .
Then these dogs were placed in a shuttlebox where they could avoid
shock by jumping over a
barrier. The shuttlebox was used to study the role of operant
conditioning in
learning. Most of the dogs failed to learn to avoid shock.
Seligman
argued that prior exposure to inescapable
shock interfered with the ability to learn in a
situation where avoidance or
escape was possible. Seligman introduced the term learned
helplessness to describe
this phenomenon.
Learned
helplessness and human depression
Seligman
argues that there are similarities
between the symptoms of depression in humans and learned helplessness
|
|
Symptoms of
depression |
Corresponding
symptom in learned helplessness |
| depressed mood |
helplessness |
| lack of interest in, and
pleasure from, almost all activities |
cognitive representation
of uncontrollability |
| decreased appetite
leading to weight loss |
helpless animals eat
less & loose weight |
| insomnia or hypersomnia |
I know of no study on
this point |
| psychomotor agitation or
retardation |
helpless animals are
passive in face of shock |
| feeling without energy |
lack of response
initiation |
| feelings of
worthlessness and guilt |
perception that
individual cannot control their environment |
| inability to think
clearly or concentrate effectively, indecisiveness |
cognitive representation
of uncontrollability |
| thoughts of death,
suicidal thoughts |
helpless animals may die
in traumatic situations |
The
motor activation deficit explanation
of learned helplessness
One
aspect of helplessness and depression
posed problems for Seligman's theory - its physiological basis.
Seligman points out
similarities between the physiological basis of depression and
helplessness:
| Physiology of
depression |
Physiology of
learned helplessness |
- Depression
is associated with a deficiency of catecholamines
(particularly norepinephrine) at central receptor sites. This is the
catecholamine theory of mood
|
- Helpless
rats have lowered levels of norepinephrine in the brain
|
Weiss
believes that 'learned helplessness' is
produced by some form of stress-induced 'debilitation' involving a
temporary reduction in
brain norepinephrine levels. He called this the motor
activation deficit hypothesis
(Weiss & Glazer, Psychosomatic Medicine, 37, p501, 1975).
You can
read a fuller account of this
controversy in Depression and Learned Helplessness
Schizophrenia: The DA theory of
schizophrenia
|
|
According
to the DA (dopamine) theory,
- schizophrenia
is associated with increased activity at dopaminergic receptor sites
- antipsychotic
drugs exert their clinical effect by reducing increased DA activity.
which
leads to predictions that:
- drugs
which increase DA activity should produce schizophrenia
- drugs
which decrease DA activity should reduce schizophrenia
|

|
Comparison of
amphetamine psychosis and paranoid schizophrenia
An
early clue that schizophrenia might
involve increased dopaminergic activity was provided by the observation
that people who
take high doses of amphetamine for many days can develop amphetamine
psychosis - a
drug-induced mental state which could be mistaken for paranoid
schizophrenia.
| Similarities
between amphetamine psychosis and paranoid
schizophrenia |
Differences
between amphetamine psychosis and paranoid schizophrenia |
| Clear
sensorium |
Strong
sexual stimulation and stereotypic compulsive behaviour in amphetamine
psychosis |
| High
incidence of auditory hallucinations |
Failure
to display flattened affect in amphetamine psychosis |
| Phenothiazines
(and other antipsychotics) are highly efficacious in treatment |
Lack
of formal thought disorder in amphetamine psychosis |
Although
it is now clear that amphetamine
psychosis and paranoid schizophrenia are not identical, the
similarities between the
states motivated research designed to explore the biochemical and
behavioural effects of
amphetamine in animals in order to understand the role of dopamine in
behaviour and to
develop medicines to treat schizophrenia.
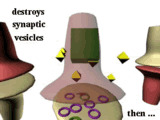
Here
are some images from a movie showing
that amphetamine releases catecholamines (especially dopamine) from
synaptic vesicles in
nerve terminals. Note that amphetamine
causes a
massive release of DA from synaptic vesicles within the presynaptic
nerve ending which
causes the postsynaptic neuron to fire.
Rationale
for animal model of
schizophrenia
One of
the first animal models of
schizophrenia involved the use of drugs that increased the release of
dopamine in the
brain. Here is the logic for an animal model that involved injecting
rats with
amphetamine.
| Rationale for the
amphetamine animal model of schizophrenia |
| 1
|
Amphetamine
induced psychosis is similar to paranoid schizophrenia |
| 2
|
Amphetamine
affects conditioned and unconditioned animal behaviour |
| 3
|
Amphetamine
releases DA from presynaptic nerve terminals |
| 4
|
Thus
the release of DA may be related to schizophrenia - this is the DA
hypothesis of schizophrenia |
| 5
|
Amphetamine-induced
psychosis is similar to 'naturally occurring' paranoid schizophrenia.
Thus DA hyperactivity may be responsible for 'naturally occurring'
schizophrenia. |
Amphetamine-induced
stereotypy as an animal model of schizophrenia
 |
An animal model of
schizophrenia has been developed which involves injecting animals
-usually rats - with amphetamine.
Amphetamine produces an increase in locomotion and an increase in
so-called stereotyped behaviour.
A behaviour is said to be stereotyped when it is
is repeated in an apparently meaningless fashion.
The cardinal features of stereotypy:
- repetition
of the behaviour
- the
invariance of the repetition i.e. the
behaviour is the same each time it is emitted
- the
apparent purposeless nature of the behaviour
are
exemplified by this cat !
|
This diagram shows that
'stereotypy' increases as a function of increasing dose of amphetamine 
- Stereotypy
rating scales express the degree of stereotypy as a single
number .
- Stereotypy
scores increase with increasing dose of amphetamine
- Stereotypy
scales ' lump-together' different behaviours
|
Amphetamine-antagonism
may detect
antipsychotic drugs
Antipsychotic
drugs such as chlorpromazine
are thought to exert their therapeutic effects by blocking DA
receptors. Here are some
images from a movie showing this effect of chlorpromazine on
postsynaptic receptors.
Notice that chlorpromazine
attaches to receptor sites
in the postsynaptic membrane normally occupied by dopamine released
from the presynaptic
neuron. This prevents dopamine activating these receptors and
consequently the
postsynaptic neuron does not fire.
You can
download the complete movie (size
c1.4MB).
A well
established screening test for novel
antipsychotic drugs involves examining their ability to antagonize the
effects of
amphetamine on animal behaviours. Here are some images from a movie
showing how chlorpromazine
may antagonize the effects of amphetamine by
blocking postsynaptic receptor sites so that
they cannot be activated by the large amount of DA released by an
injection of
amphetamine.
In this
simulation chlorpromazine is injected
first and occupies postsynaptic receptor sites. Then amphetamine is
injected. This mimics
the arrival of an action potential at the nerve ending and causes the
release a large
number of DA molecules from the presynaptic neurone. But this released
DA cannot activate
the postsynaptic receptors because they are occupied by chlorpromazine
molecules.
Consequently, the postsynaptic neurone does not generate an action
potential. The released
DA is broken down by enzymes in the synaptic cleft, or is reabsorbed
into the presynaptic
neurone.
You can
download the complete
movie (size c1.8MB).
You can
read a fuller account of the DA
theory of schizophrenia
Anxiety:
The
Geller-Seifter paradigm as an animal model of
anxiety
The Geller-Seifter
paradigm was developed to screen drugs for their ability to control
human anxiety. The technique involves training rats in a Skinner box on
two different schedules of reinforcement.
- Under
the VI (variable interval) schedule, each
bar-press is reinforced by sweetened milk at irregular intervals.
- Under
the CRF (continuous reinforcement) schedule every
response is reinforced by the delivery of sweetened milk, but in
addition, each response is punished by the delivery of a brief,
inescapable electric shock to the animal's feet.
The
switch from VI to CRF is signalled by a tone or light. The CRF schedule
may produce 'anxiety' in the rat by placing it in a 'conflict'
situation.
|
 |
 |
This
diagram shows the performance of a rat in the Geller-Seifter paradigm
after a saline injection (upper panel) and
following the injection of a benzodiazepine (lower
panel) .
The
effect of the benzodiazepine is revealed in performance on the CRF
schedule of reinforcement where the rat is presented with a conflict
between bar-pressing for a guaranteed reward, but at the cost of an
inevitable electric shock to its feet
Important
points to note:
- the
drug does not affect performance on the VI component
- performance
on the CRF (punished) schedule is increased by the anti-anxiety drug
| |
|
Schedule of
reinforcement |
| |
|
VI
(unpunished) |
CRF
(punished) |
| Injection |
Saline |
high,
stable response rate |
no
responding |
| Benzodiazepine |
no
change to high, stable response rate |
increased
response rate |
|
Correlation of
rat punishment potency with clinical potency
 There is a very strong
correlation ( r=.987 )
between the dose of drug that needs to be given to a patient to control
their anxiety, and
the dose of the same drug that reduces conflict in animals.
There is a very strong
correlation ( r=.987 )
between the dose of drug that needs to be given to a patient to control
their anxiety, and
the dose of the same drug that reduces conflict in animals.
In this
diagram
- the
minimum laboratory dose represents the lowest dose
producing significant anti-conflict effects in an animal model
- average
clinical daily dose represents dose found
effective in treating patients in over 100 studies. Redrawn from data
in Sepinwall & Cook, 1975.
This
suggests that there may be a common
biochemical basis for the effect of the drugs in humans and animals.
One of the first
theories to explore this idea was Stein's theory that the anti-anxiety
effect of
benzodiazepine drugs involved the serotoninergic system in the brain.
Refer
to an earlier lecture on anxiety for
discussion of the specificity of benzodiazepine effects on the
Geller-Seifter paradigm.
Seminar discussion topics
Read
Hitzeman (2000) Animal
models of psychiatric disorders and their relevance to alcoholism which
is
available online to familiarize yourself with the following
concepts and techniques:
| Face
validity
phenotypes and
endophenotypes
prepulse inhibition of the acoustic startle response
Predictive
validity
Etiological
validity
Genetic
validity
Reliability
- inter-laboratory
reproducibility
- test-retest
reliability; carry-over effects
|
Animal
models of schizophrenia
- prepulse inhibition
- latent inhibition
- catalepsy
- hallucinogens
- acute and chronic
amphetamine -drug sensitization
- NMDA:
N-methyl-D-aspartate
Animal
models of depression
- reserpine
- learned helplessness
- behavioural despair
Animal
models of anxiety and fear
- conditioned and
unconditioned fear
- open-field test
- light-dark transition
- elevated plus maze
- fear potentiated
startle
|
Read Geyer
and Markou (2000) Animal Models of Psychiatric
Disorders: which
is available online
and consider the following points:
- What
are the limitations of animal models of psychiatric disorders? How may
they be overcome?
- Describe
the different types of animal model.
- Are
the philosophical roots of behavioural pharmacology in ethology,
evolutionary psychology, psychobiology or behaviourism?
- Describe
an animal model which is theoretically based on psychological
constructs.
- Describe
an animal model which is theoretically based on pharmacological
constructs.
- What
is meant by the terms 'analogy' and 'homology' in physiological and
behavioural characteristics? Why are these terms important to
behavioural pharmacology?
- Explain,
with examples drawn from your reading, the phrase 'reliable animal
model'.
- "Mirror,
mirror on the wall, who is the prettiest of them all?" Is 'predictive'
the prettiest of all the validities?
- What
are the most important properties of an animal model?
- To
what extent is construct validity ephemeral?
- Does
the learned helplessness model have etiological validity?
- What
factors limit the development of models with etiological validity?
- Is
etiological validity desirable?
- Will
animal models based on genetic manipulations to produce animals with
altered neurotransmitter receptor characteristics provide useful models
of psychiatric conditions?
- Provide
examples of the definition of a construct through convergent
operationalism .
- Does
'face validity' provide any value to an animal model?
- Evaluate
stress-induced behavioural changes as animal models of depression.
- Explain
the term 'false positive'.
- What
is anhedonia and how can it be studied and modelled in animals?
- Describe
the effects of stress on reward systems in the brain.
- Evaluate
psychostimulant models of schizophrenia.
- Explain
the terms tolerance and sensitization.
- Evaluate
animal models of schizophrenia based on sensorimotor gating.
- Compare
and contrast sensorimotor and psychostimulant models of schizophrenia.
Read Barrett
and Miczek (2000) Behavioral Techniques in Preclinical
Neuropsychopharmacology
Research which is available
online and consider the following points:
- Discuss,
with examples, the aims of behavioural pharmacology.
- What
does the study of behaviour contribute to pharmacology?
- Discuss
the contribution of the study of conditioned and unconditioned
behaviour to behavioural pharmacology
- How
can the effects of drugs on species-specific behaviours be objectively
measured?
- Describe,
with examples drawn from your reading, how drugs affect behaviour
maintained by schedules of reinforcement.
- What
has the use of drugs as discriminative stimuli told us about the
biological basis of fear?
- How
useful are 'simple' behavioural tests in the discovery of novel
psychoactive drugs?
References
and
supplementary resources
- American College
of Neuropsychopharmacology (ACNP) website
- Barrett and
Miczek (2000) Behavioral Techniques in Preclinical
Neuropsychopharmacology Research: In Psychopharmacology - The Fourth
Generation of Progress. The American College of
Neuropsychopharmacology. Available online.
- Beans (1999)
Creative animal models screen drugs .APA Monitor Online VOLUME 30,
NUMBER 10 November 1999. Available
online
- Bronski
(ND). The Destiny of Biology . An
interview with the social constructionist Anne Fausto-Sterling author
of Myths of Gender:
Biological Theories about Women and Sexing the Body: Gender Politics
and the Construction of Sexuality and Men. Available
online
- Geyer
and Markou (2000) Animal Models of
Psychiatric Disorders: In Psychopharmacology - The Fourth
Generation of Progress. The American College of
Neuropsychopharmacology. (ACNP website) Available online
- Hitzeman (2000).
Animal models of psychiatric disorders and their relevance to
alcoholism. Alcohol Research & Health, 24/3, 149-158. Available online
- Koob (2000)
Animal Models of Drug Addiction: In Psychopharmacology - The Fourth
Generation of Progress. The American College of
Neuropsychopharmacology. (ACNP website) Available online
- Russell
et al (1990). Behavioral Measures of
Neurotoxicity .
National Academic Press, Washington. Available online
- SALMON
website
- Szechtman
et al (2001). Compulsive checking behavior of
quinpirole-sensitized rats as an animal model of Obsessive-Compulsive
Disorder (OCD): form and control. BMC Neuroscience, 2:4. Available online
- University of
California at Los Angeles (ND). UCLA Research
Application Tracking System. Provides an insight into the regulation of
research involving animals at UCLA. Available
online
Copyright Dr.
C.A.P. Kenyon 1994-2006

 This picture gives an
idea of the facilities available in an operant chamber, or Skinner box.
This picture gives an
idea of the facilities available in an operant chamber, or Skinner box.

 The relative angles of
successive slopes gives a
visual indication of the regularity in response rate. Steep slopes
reflect high response
rates and vice versa.
The relative angles of
successive slopes gives a
visual indication of the regularity in response rate. Steep slopes
reflect high response
rates and vice versa. 










 For example, the library
has us all
on a Sidman avoidance schedule. You can borrow a book for 14 days. If
you take it back
before the loan period is up, you can sign it out for another 14 days.
If you don't take
it back within 14 days you start to get fined. Every day you keep the
book beyond the loan
period the fine rises. Thus the library is operating a 14 day response-shock
(borrow-return)
interval, and a 1 day shock-shock (fine-fine)
interval. A smart person who was a
slow reader would return the book to the library every 13-14 days and
keep renewing the
loan period.
For example, the library
has us all
on a Sidman avoidance schedule. You can borrow a book for 14 days. If
you take it back
before the loan period is up, you can sign it out for another 14 days.
If you don't take
it back within 14 days you start to get fined. Every day you keep the
book beyond the loan
period the fine rises. Thus the library is operating a 14 day response-shock
(borrow-return)
interval, and a 1 day shock-shock (fine-fine)
interval. A smart person who was a
slow reader would return the book to the library every 13-14 days and
keep renewing the
loan period. 



























 There is a very strong
correlation ( r=.987 )
between the dose of drug that needs to be given to a patient to control
their anxiety, and
the dose of the same drug that reduces conflict in animals.
There is a very strong
correlation ( r=.987 )
between the dose of drug that needs to be given to a patient to control
their anxiety, and
the dose of the same drug that reduces conflict in animals.