Overview:
A
quick glance at the title might lead you to believe that this is going
to be a very interesting lecture. Well it is interesting, but not in
the way you think!
You
will see how the role of hormones in behaviour has been elucidated by
removing the endocrine glands, observing the animals behaviour and then
replacing hormones to reverse the effects of the original intervention.
This is a fundamental scientific strategy (remove - observe - replace -
observe) and crops up in many behavioural experiments.
A
very important question is: Do the effects of these hormone
manipulations reflect an effect of hormones on the animal's brain, or
are the effects secondary to changes in peripheral tissues? It would be
nice to believe that we were observing central effects - after all we
are principally interested in the brain. But the possibility that the
animal's behaviour is affected by more mundane peripheral effects needs
to be experimentally verified. Studies which address this issue are
described.
|
Learning objectives
After
reading this material you should be able to:
- describe
the sexual behaviour of female rats
- operationally
distinguish between receptivity, proceptivity and attractiveness
- describe
the sexual behaviour of male rats
- list
4 measures of male rat sexual performance
- distinguish
between time limited, series limited and satiation tests of sexual
behaviour
- draw
a diagram to illustrate the effects of estrogen injection dose on
sexual behaviour in female adult rats
- describe
the effect of ovariectomy & estrogen replacement on rat uterine
weight
- describe
the effects of castration on sexual behaviour of male rodents
- describe
the effect of castration and testosterone replacement on seminal
vesicle weight and papillae of glans penis in male rats
- explain
the importance of studies involving central injection of
hormones
|
Hormones and human male
sexual behavior
|
Heim
and Hursch (1979), reviewed the effects
of castration on men convicted of sex crimes.
- 50+%
stopped exhibiting sexual behavior shortly after castration
- about
25% showed a gradual decline in sexual behaviour
- around
10% showed no change in sexual behaviour after castration
Similar
effects have been shown in rodents.
There are significant individual difference between animals in their
sexual behaviour
which are not related to circulating level of testosterone and which
persist after
castration and hormone replacement therapy (see Nelson, 1995)
|
|
|
Davidson
et al (1982), studied the effects of androgen injections in men who
had very low levels of testosterone circulating in their bodies (called
hypogonadal
men).
Six patients each received:
- 100
mg of testosterone enanthate ( a long lasting androgen) dissolved in
oil
- 400
mg of testosterone enanthate dissolved in oil
- placebo
(the oil vehicle used to dissolve the androgen)
This
study used a within-subjects design;
each patient received each treatment. There was a gap of six weeks
between treatments, and
the order of treatments varied between patients to control for
treatment order (carry
over) effects.
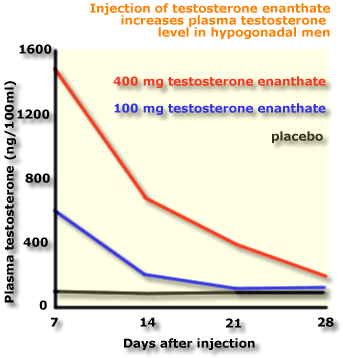
The
diagram shows that injection of
testosterone enanthate increased plasma testosterone levels. Notice
that the effect is
dose-dependent, and temporary. Peak effects were seen seven days after
injection.
|
 |
|
 |
The
hypogonadal men kept daily
diaries of their sexual behaviour and penile erections. The biggest
effects of
testosterone injections were reported one week after injection. This
corresponds to the
time of peak effect on plasma testosterone level.
This
diagram shows these effects. Notice that
the effects are dose-dependent, and that the values do not reach 100%
for any of the
measures, even at the highest dose of testosterone (400 mg).
|
|
| Of
course one possibility
is that not enough testosterone was injected. This is unlikely because
studies indicate
that plasma testosterone levels as high as 1500 ng/100 ml (the level
seen after injection
of 400 mg testosterone enanthate) are rare in normal men.
In
fact
the most surprising thing about male
testosterone level is its huge range - shown in red in this diagram .
In general there is
a tendency for testosterone level to increase during teenage years and
the 20s. it usually
remains steady in middle life, but after 60 there is a decline to the
level seen in young
boys. Nevertheless as this diagram shows there are a lot of 90 year old
men out there with
teenage levels of circulating testosterone! (Vermeulen et al, 1972).
Global
Variation in Male Testosterone and Age by Peter T. Ellison
et al at Harvard contains a
nice diagram showing the decline in male salivary testosterone level as
a function of age.
|

|
|
Frank
Beach
 |
In order
to understand the role of hormones in our behaviour scientists have
conducted fundamental research using laboratory animals. Frank
Beach is generally considered to be the founding father of the study of
the effects of hormones on behaviour - an area of scientific
investigation that is known today as behavioural endocrinology
or psychoendocrinology. His first book, Hormones and Behavior (1948),
summarizes all that was known at the time and organized the field for
all subsequent investigators. Beach devoted his whole life to basic
research and justified his endeavours as follows:
"To
what degree should my choice of research work be governed by human
needs, by social imperatives, and how am I going to justify spending
all of my energies on any research that does not bear directly on
pressing human problems.... The solution, or rationalization, that I
have finally come up with is that it is a perfectly worthwhile way of
spending one's life to do your level best to increase human knowledge,
and it is not necessary nor is it always even desirable to be
constrained by possible applicability of what you find to immediate
problems. This may sound very peculiar to some young people, but it is
a value judgement which I myself have made and which I can live with."
As you probably realize, Frank Beach is my hero, and I have spent a
large part of my research career following up his fascinating work on
the role of sensory systems in controlling reproductive behaviours.
Point
to ponder
Why are you interested in the study of behaviour?
Do you think it would be possible to pursue a research career in the
1990s along the lines followed by Beach?
What do you think is the general publics' perception of science today,
and why? |
|
Sexual
behaviour in the female rat
 The estrus
cycle lasts four or five days in
the rat, and is associated with variations in the level of the hormones
estrogen
and progesterone released by the ovaries.
Around mid-cycle there is a 12-15
hour period of estrus or 'heat' during which the female is receptive
to the male.
Estrus is timed to occur when eggs are released from the ovaries
(ovulation) to increase
the likelihood that they will be fertilized by sperm from the male rat.
The estrus
cycle lasts four or five days in
the rat, and is associated with variations in the level of the hormones
estrogen
and progesterone released by the ovaries.
Around mid-cycle there is a 12-15
hour period of estrus or 'heat' during which the female is receptive
to the male.
Estrus is timed to occur when eggs are released from the ovaries
(ovulation) to increase
the likelihood that they will be fertilized by sperm from the male rat.
At one
time it was thought that females were
'passive recipients' of male ardour. Many early studies measured female
sexual behaviour
in terms of 'lordosis'. Lordosis refers to a
characteristic posture in which the
female rodent arches her back and moves her tail to permit penetration
by the male.
Lordosis is a reflex which usually occurs when a
male grasps the female's flanks.
Points
to ponder
 Why do you think - at
one time - female rats were viewed as passive recipients of male sexual
sexual behaviour? Why do you think - at
one time - female rats were viewed as passive recipients of male sexual
sexual behaviour?
What does this tell us about the effect of the influence of
researchers' attitudes and values?
Can you think of other examples of this effect? |
Because
lordosis is elicited by males this
gives the impression that females are passive players in a sexual
encounter. However, the
female rat often plays a very active role in initiating copulation. If
she is in estrus
she may:
- approach
the male, nuzzle him and sniff his genitals
- wiggle
her ears
- hop
and dart around the cage
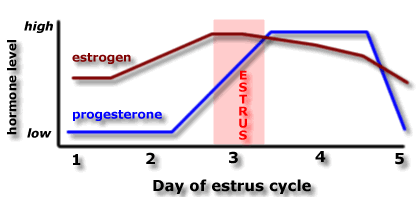 In the
first part of this lecture we
will examine the role of estrogen and progesterone in the sexual
behaviours of female
rats. This diagram shows that estrogen and progesterone levels increase
before ovulation,
and are at a relatively high level during estrus. A number of
experiments have examined
the roles of estrogen and progesterone on the sexual behaviours of
female rats.
In the
first part of this lecture we
will examine the role of estrogen and progesterone in the sexual
behaviours of female
rats. This diagram shows that estrogen and progesterone levels increase
before ovulation,
and are at a relatively high level during estrus. A number of
experiments have examined
the roles of estrogen and progesterone on the sexual behaviours of
female rats.
These
studies employ the hormone-replacement
strategy. Female rats have their ovaries removed under general
anaesthetic in an operation
called ovariectomy.
A
female rat who has lost here ovaries in
this way is said to be ovariectomised. Male rats do
not mate with ovariectomised
females, presumably because they do not come into estrus (heat).
This
may be a consequence of the loss of
estrogen and progesterone which are normally secreted by the ovaries.
To investigate this
possibility, ovariectomised rats are injected with estrogen or estrogen
and progesterone
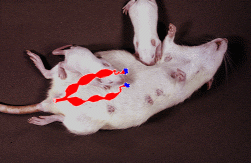
before being given a test of their sexual behaviour. A diagram of the
approximate location
of the ovaries and uterus has been superimposed on this photograph -
Ignore the suckling
pups.
Effects
of estrogen on sexual behaviour in female rats

In this experiment (Davidson et al, 1968) adult female rats with
ovaries removed
(ovariectomy) were given daily injections of estradiol benzoate.
Five
groups each received a different dose
over a 12 day period.
On days
8, 10 and 12 each rat was given about
10 test trials with a sexually active male.
The
behavioural measure was the lordosis
score, that is, the percentage of trials on which the female showed a
lordosis response
when the male attempted to mount her.
Scores
are mean performance over the three
test days.
 Effects of progesterone
on sexual
behaviour in female rats
Effects of progesterone
on sexual
behaviour in female rats
Davidson's
experiment tells us that estrogen
replacement restores one aspect of the sexual behaviour of
ovariectomised rats. But his
experiment involved chronic treatment with estrogen
over several days. Normally
estrogen levels increase a matter of hours before estrus. It could be
said that chronic
experiments do not tell us much about the role of hormones under
natural conditions.
Therefore subsequent studies explored the acute
effect of estrogen, and the
interaction between estrogen and progesterone.
A study
by de Jonge et al shows that a single
injection of estrogen can elicit lordosis behaviour and that the effect
of estrogen is
roughly dose dependant - the greater the dose of
estrogen injected the higher the
females lordosis score.
Perhaps
the more interesting finding is that
progesterone facilitates the effect of estrogen. If ovariectomised rats
are injected with
progesterone, they do not show lordosis. But this diagram shows that if
progesterone is
given in conjunction with estrogen, the lordosis score was increased
compared to the score
under estrogen alone.
Measuring
sexual motivation in the female
rat
In the
experiments considered so far, only
one aspect of female sexual behaviour was explored - the lordosis
response. But we now
know that even female rats can be strongly motivated
to engage in sexual behaviour,
and can play an active role in initiating sexual
behaviour from males. The article
by Doty (1974) was seminal in bringing about this change in attitude
towards sexuality in
female rats.
In fact
hormones have at least three effects
on female sexual behaviour. Hormones modify:
- Receptivity:
the females ability to copulate. The lordosis score measures this by
expressing the number of times a female emits the lordosis posture as a
percentage of the number of times she is mounted by a sexually
experienced and vigorous male. Notice that lordosis does not measure
the female's willingness to copulate. Indeed the small test cages used
in most studies prevent females escaping from sexually vigorous males.
- Proceptivity:
describes the female's eagerness to copulate with a male. This can be
assessed by recording the extent to which females approach and spend
time with males, and emit female sexual behaviours such as hopping,
darting, earwiggling, investigatory and presenting behaviours designed
to initiate sexual interest in males.
- Attractiveness:
a measure of how attractive a female is to a male. This is assessed by
measuring how willing males are to copulate with particular females.
For example, male rats will not attempt to copulate with ovariectomised
females. This deficit in attractiveness can be overcome by estrogen and
progesterone replacement therapy
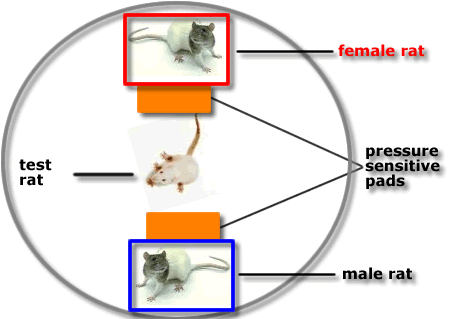 This diagram shows a
piece of
apparatus that can be used to measure female sexual motivation.
A male and a female
rat are enclosed in individual boxes with gauze windows placed within a
larger test
apparatus.
This diagram shows a
piece of
apparatus that can be used to measure female sexual motivation.
A male and a female
rat are enclosed in individual boxes with gauze windows placed within a
larger test
apparatus.
The
time spent by a test rat in front
of each box is recorded through pressure-sensitive pads located outside
each small box.
If a
female test rat spends more time in
front of the box containing a male rat than close to a female, this
suggests that the test
rat is sexually motivated and exhibiting proceptive
behaviour.
If a
male test rat spends more time in front
of a box containing a female in estrus, than in front of an
ovariectomised rat, this
suggests that he finds the estrus female more attractive.
Effects
of hormones on female proceptive behaviour
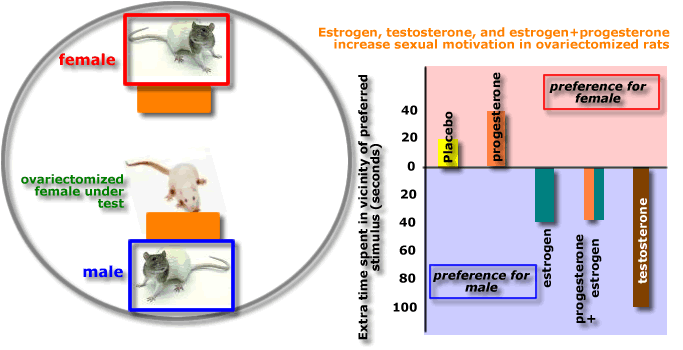
This
picture shows the results of an
experiment which examined the effects of various hormones on the proceptive
behaviour of female rats.
Injection
of estrogen, testosterone or a
combined injection of estrogen and progesterone, increased the amount
of time
ovariectomised rats spent in the vicinity of a male rat. This suggests
that these hormones
control proceptive behaviour in the female rat.
In this
study de Jonge et al measured the
amount of time ovariectomised rats spent in front of cages containing
male and female rats
during a 15 minute test.
I have
expressed their results as the extra
amount of time spent in front of the preferred cage. For example, after
an injection of
testosterone the ovariectomized rats spent on average 100 seconds
longer in front of the
cage holding the male rat compared to the amount of time spent in front
of the cage
containing the female.
There
are two interesting results in this
experiment:
- testosterone
- a hormone we normally consider involved in male sexual behaviour -
increased proceptive behaviour in females. The adrenal glands are a
source of testosterone in males and females.
- progesterone
did not facilitate the effect of estrogen on proceptive behaviour.
Contrast this to the facilitative effect of progesterone on
estrogen-induced lordosis
One
explanation for this effect of
testosterone involves conversion of testosterone into estrogen by a
process called aromatization.
This is unlikely because, in a further experiment de Jong et al, showed
that a synthetic
testosterone - that cannot be converted to estrogen also increased
proceptive behaviour in
ovariectomised rats.
Testosterone
may also be involved in human
sexual motivation (Carlson, 2001).
Point
to ponder
How might you measure the effect(s) of hormones on human sexual
behaviour? |
Effect
of estrogen on growth of rat uterus
 How might
estrogen affect sexual behaviour? It's
tempting to conclude from the results above showing the effect of
estrogen on sexual
receptivity that estrogen directly stimulates brain cells that control
behaviour.
How might
estrogen affect sexual behaviour? It's
tempting to conclude from the results above showing the effect of
estrogen on sexual
receptivity that estrogen directly stimulates brain cells that control
behaviour.
Alternatively
the hormone may be having an
effect on sensory stimulation from peripheral tissue that changes
behaviour without the
hormone affecting the brain directly. For example the diagram shows the
effect of
ovariectomy on rat uterus. Compared to a normal uterus, the uterus in
an ovariectomised
rat is small and shrivelled. Uterine weight recovers under estrogen
replacement therapy.
Can the
decline in sexual activity in
ovariectomised animals be simply explained as due to pain when the
animal is mounted by
the male? Another possibility is that ovariectomised animals do not
emit scent signals (pheromones)
that make them attractive to male animals.
These
simply explanations need to be ruled
out before we can conclude that the effects of hormones seen in the
behavioural experiment
involve direct effects of hormones on the brain.
Effects
of central injection of hormones on sexual behaviour:
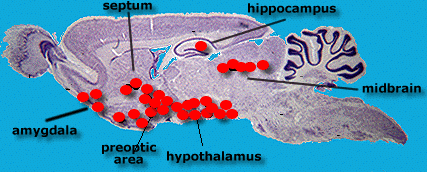 There are specific receptor sites in the brain
that act as target cells for
hormones released by the testes and ovaries. These are shown as red
blobs in the diagram.
There are specific receptor sites in the brain
that act as target cells for
hormones released by the testes and ovaries. These are shown as red
blobs in the diagram.
Receptor
sites are particularly concentrated
at the base of the brain in an area running from the preoptic
area back to the hypothalamus
( but note that several other areas including the midbrain and
hippocampus contain hormone
receptor sites.)
Injecting
estrogen into the hypothalamic area
of ovariectomised female rats restores sexual receptivity. This
procedure involves
lowering a hollow tube ( called a cannula) containing hormone into the
brain.
Implanting
these hormones into other parts of
the brain does not restore sexual behaviour which shows that the
effects are site
specific. Furthermore the brain implants are not associated with
recovery of peripheral
tissue that is hormone sensitive, which indicates that the hormone has
not leaked into the
animals circulation, and supports the conclusion that the restoration
of behaviour is the
result of the hormone affecting brain tissue that mediates sexual
behaviour in normal
animals.
How
hormones affect the brain to control
sexual behaviour is the subject of a separate lecture on your course.
Sexual
behaviour in the male rat
 Sexual
behaviour in male rats consists of three
behaviours:
Sexual
behaviour in male rats consists of three
behaviours:
- Mount:
the animal assumes the copulatory position on top of the
female and grasps her flanks, but does not not insert his penis into
the female's vagina
- Intromission:
the male mounts the female and briefly (200-300 milliseconds) inserts
his penis. . Semen is not released during intromissions or mounts.
- After
10 to 12 intromissions spaced 20-30 seconds apart, the rat ejaculates
semen.
It can
be very difficult - even for a trained
observer - to distinguish between these behaviours. There are subtle
differences in the
style of dismounts that help distinguish between mounts and
intromissions. After an
ejaculation, the male tends to pause longer before dismounting. Every
ejaculation is
preceded by a number of mounts and intromissions. Typically rats have
multiple
ejaculations before they reach exhaustion (satiation).
After each ejaculation there
is a refractory period lasting several minutes. Sexual encounters are
divided into a
number of 'series' depending upon the number of
ejaculations. A series starts with
a mount or intromission, and ends with an ejaculation.
Numerous
measures of the relationships
between mounts, intromissions and ejaculations are recorded to measure
sexual motivation
and performance. For example:
- Mount
Latency, the time that elapses between introducing the male
and female to the test apparatus before the male mounts the female
- Intermount
Interval: the time between successive mounts
- Inter
Intromission Interval: the time between successive
intromissions
- Post
ejaculatory Interval: the time elapsing between an
ejaculation and next mount or intromission, i.e. the beginning of the
next copulatory series
This list of
behavioural measures is by no means exhaustive.
Tests
of sexual behaviour are either:
- Time
limited: the experiment ends after an arbitrary number of
minutes
- Series
limited: the test ends after an arbitrary number of
ejaculations
- Satiation
criterion: the test ends after the passage of an arbitrary
period of time without a mount, intromission or ejaculation
A
model of male sexual motivation
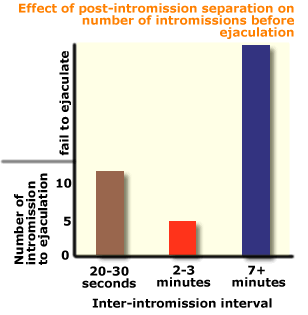 Under
normal conditions male rats typically emit an intromission
every 20 to 30 seconds, and ejaculate after 10-12 intromissions.
Under
normal conditions male rats typically emit an intromission
every 20 to 30 seconds, and ejaculate after 10-12 intromissions.
The
Swedish psychologist Knut Larsson
discovered that if the male was removed from the test area after each
intromission, and
then replaced 2-3 minutes later, the males ejaculated after fewer
- four or five -
intromissions.
However,
if the males were removed for seven
or more minutes, they needed a large number of intromissions to achieve
ejaculation, and
some failed to ejaculate at all.
Larsson
suggested an interesting theory to
account for this finding whichexplains the role of intromissions in rat
sexual behaviour.
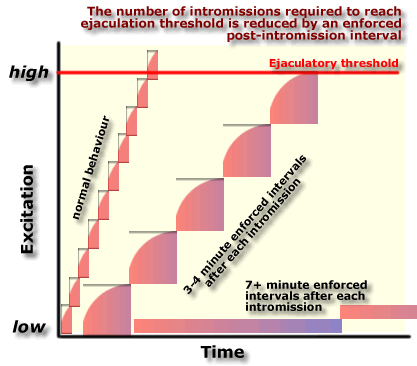
| According to Larsson's "accumulating
excitation" model the excitation required for a rat to
ejaculate increases for several minutes after each intromission, and
then declines when there is a longer interval after intromission.
This
model can explain why fewer intromissions are required to reach an
ejaculation if the rat is prevented from intromitting for 2-3 minutes
after his previous intromission. Each successive intromission builds on
the accumulated excitation from preceding intromissions.
However
if the enforced interval is too long (e.g. 7 minutes) between
successive intromissions, excitation may never build up to the level
required to trigger ejaculation.
The
next diagrams show how intromissions increase excitation leading up to
ejaculation.
|
 |
 |
Effects of
castration on sexual behaviour of male rodents
 In the
experiment shown here, carried
out on male guinea pigs (Grunt & Young, 1952), sexual behaviour
decreased shortly
after castration (gonadectomy) and was re-established by the
administration of
testosterone to castrated animals.
In the
experiment shown here, carried
out on male guinea pigs (Grunt & Young, 1952), sexual behaviour
decreased shortly
after castration (gonadectomy) and was re-established by the
administration of
testosterone to castrated animals.
- Animals
in the Intact control group were untreated during
the 41 weeks of the experiment
- Animals
in the Castrate control group were castrated in
week 10. Notice the gradual decline in mating behaviour. This contrasts
with the rapid loss of female receptivity following ovariectomy.
- Animals
in the Experimental group were castrated in week
10, and then injected with testosterone proprionate (25 microgram / 100
gm body weight / day) each day during weeks 26 through 35. In weeks 35
through 41 the daily dose of testosterone was increased to 50 microgram
/ 100 gm body weight. Notice that increasing the dose of testosterone
did not increase sexual performance above that seen in intact control
subjects. The gradual recovery of mating under testosterone replacement
therapy mirrors the slow decline in sexual behaviour after castration.
The
hands on the figure point to weeks when
these treatment changes occurred.
The role of
estrogens in male sexual behaviour
Estrogen
and androgen are names given to two
important families of hormones. To complicate
things further many brain cells
contain an enzyme that converts androgens such as testosterone into
estradiol (an
estrogen). this process is called aromatization.
Although it is convenient to refer
to androgens and estrogens as 'sex hormones' it is important to point
out that males and
females produce both types of hormone. Furthermore, sex hormones can be
produced in the
adrenal glands as well as the gonads (testes and ovaries).
We have
already seen that testosterone plays
an important role in female
sexual motivation. It
appears that estrogen is important for maintaining
mating in males.
This
table shows that only androgens that can
be converted to estrogens restore sexual behaviour in castrated male
rodents. Furthermore
estradiol (an estrogen) also activates mating in castrates.
| Hormone |
Aromatized to estrogen? |
Maintains sexual
behaviour
in castrated males? |
| testosterone |
 |
 |
| androstenedione |
 |
 |
| dihydrotestosterone |
 |
 |
| estradiol |
 |
 |
Dihydrotestosterone
is thought to play an
important role in maintaining the sensitivity of the penis (see Nelson,
1995)
Point
to ponder
If you were called as an expert witness in the trial of a pedophiliac,
what treatment would you recommend? |
Effect
of testosterone on seminal vesicle
weight
 It is
tempting to think that the restoration of
sexual behaviour in castrated male animals given testosterone
replacement therapy is the
result of the hormone reactivating 'centers' in the
brain that control sexual
motivation.
It is
tempting to think that the restoration of
sexual behaviour in castrated male animals given testosterone
replacement therapy is the
result of the hormone reactivating 'centers' in the
brain that control sexual
motivation.
This is
an attractive hypothesis because it
implies that these experiments will tell us something important about
the relationship
between brain and behaviour.
However
a more parsimonious explanation might
be that castration somehow affects tissue outside the brain that is
involved in sexual
behaviour. For example, this diagram shows the effect of castration and
testosterone
replacement on seminal vesicle weight.
Effect of hormones
on papillae of glans penis in male rats
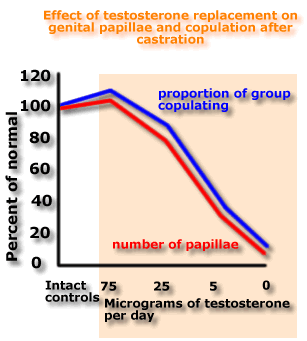 If you are
still not convinced by this argument that
recovery of sexual behaviour with testosterone replacement is the
result of recovery of
peripheral function, consider this diagram which shows the effect of
castration and
testosterone replacement therapy upon the number of genital papillae
and the number of
animals continuing to copulate four weeks after castration.
If you are
still not convinced by this argument that
recovery of sexual behaviour with testosterone replacement is the
result of recovery of
peripheral function, consider this diagram which shows the effect of
castration and
testosterone replacement therapy upon the number of genital papillae
and the number of
animals continuing to copulate four weeks after castration.
The
glans penis (tip of the penis) in the rat
is covered in sensitive papillae. Maybe it's the recovery of sensation
in this area that
is responsible for the recovery of behaviour under testosterone
therapy. All scores are
expressed as percentages of the averages for normal rats (Data from
Beach & Levinson,
1950).
This
experiment suggests that the effects of
replacement testosterone on sexual behaviour could be mediated by
changes in the
sensitivity of the rats' penis, rather than an effect of the hormone on
brain tissue.
Although
dihydrotestosterone is thought to
play an important role in maintaining the sensitivity of the penis (see
Nelson, 1995), it
is now clear that some of the effects of testosterone on behaviour
involve the brain. This
is a complex area and we only have time in this lecture to give a
flavour of the research
which led to this conclusion.
Effects
of central injection of hormones on male sexual behaviour:
 There are specific receptor sites in the brain
that act as target cells for
hormones released by the testes and ovaries. These are shown as red
blobs in the diagram.
There are specific receptor sites in the brain
that act as target cells for
hormones released by the testes and ovaries. These are shown as red
blobs in the diagram.
Receptor
sites are particularly concentrated
at the base of the brain in an area running from the preoptic
area back to the hypothalamus
( but note that several other areas including the midbrain and
hippocampus contain hormone
receptor sites.)
Injecting
testosterone into the hypothalamic
area of castrated male rats restores their sexual receptivity. This
procedure involves
lowering a hollow tube ( called a cannula) containing hormone into the
brain.
- Implanting
testosterone into other parts of the brain, or
- injecting
cholesterol
does
not restore sexual behaviour.
Cholesterol
is used as a control substance in
these experiments because it has a similar chemical structure to
testosterone. Cholesterol
is converted into testosterone by enzymes in the gonads and adrenal
glands. These enzymes
are not present in brain tissue. Therefore cholesterol is a very good
control, or placebo,
in this type of experiment.
These
experimental results indicate that the
effects of testosterone are site specific. Furthermore the brain
implants are not
associated with recovery of peripheral tissue that is hormone sensitive
(e.g. seminal
vesicle weight). This indicates that the hormone has not leaked into
the animals
circulation, and supports the conclusion that the restoration of
behaviour is the result
of the hormone affecting brain tissue that mediates sexual behaviour in
normal animals.
Hypothalamic
implants of estrogen restore
sexual behaviour in male castrates. This suggests that the effect of
brain testosterone
implantation involves aromatization.
How
hormones affect the brain to control
sexual behaviour is the subject of a separate lecture on your course.
References
- Carlson
(2001). Physiology of Behavior, 7th Edition, Allyn and Bacon, Boston.
- Davidson
et al. (1968). Endocrinology, 82, 193.
- Davidson
et al (1982). J. Clin. Endocrinol. Metab., 11, 599-623.
- de
Jonge, Burger, and van de Poll, Acute effects of gonadal hormones on
lordosis behavior of the female rat. Behavioural Brain Research.
- de
Jonge, Eerland, and van de Poll, The influence of estrogen,
testosterone and progesterone on partner preference, receptivity and
proceptivity. Physiology and Behavior.
- de
Jonge, Kalverdijk and van der Poll, Androgens are specifically
implicated in female rat sexual motivation. Pharmacology, Biochemistry
and Behavior.
- Doty
(1974),. A cry for the liberation of the female rodent: courtship and
copulation in rodentia. Psychological Review, 81, 159-172.
- Heim
and Hursch (1979). Archives of Sexual Behaviour, 8, 281-304, 1979.
- Nelson
(1995), An Introduction to Behavioural Endocrinology,
Sinauer,Sunderland, Mass.
- Vermeulen
et al, (1972). J. Clin. Endocrinol. Metab.,34, 730-735.
Supplementary
material
HEFCE, the
funding body for universities and colleges for the UK, has purchased a
3 year license to IDEAL,
the Academic Press online journal library. If you are a member of a UK
academic institution (i.e. HEFCE funded) you now have full access
rights to this online library which enables you to read the full text
of articles in Academic Press journals. Note IDEAL
uses your computer's IP internet address to allow access. Consequently
you may not gain automatic access if you are a HE student using a PC at
home.
The following articles cover topics raised in the lecture in greater
depth:
- Pfaus,
FRANK BEACH AWARD. Homologies of animal and human sexual behavior.
Hormones and Behavior, 30,3,187-200,1996. Follow this link to IDEAL
- V.
D. Ramirez,J. Zheng ,Membrane Sex-Steroid Receptors in the Brain,
Frontiers in Neuroendocrinology, 17, 4, 402-439,1996. Follow this link
to IDEAL
Other
online resources
- Global
Variation in Male Testosterone and Age by Peter T. Ellison et
al at Harvard contains a nice diagram showing the decline in
male salivary testosterone level as a function of age.
- NEERAJA
SANKARAN,
The Science Of Sex: What Is It And Who's Doing It? The
Scientist, Volume 8, #6, page 3, 21 March 1994
- Archives
of Sexual Behavior, Volume 27 Number 1 Feb 1998. 'Pheromonal Influences
on Sociosexual Behavior in Men' Winnifred B. Cutler Erika Friedmann
Norma L. McCoy
The Feb 1998 issue of Archives of
Sexual Behavior is available free of charge. According to the
authors "Significantly more pheromone than placebo users
increased above baseline in sexual intercourse and sleeping with a
romantic partner. There was a tendency for more pheromone than placebo
users to increase above baseline in petting/affection/kissing, and
informal dates, but not in self-stimulation to ejaculation or in formal
dates . These initial data need replication but suggest that human male
pheromones affected the sexual attractiveness of men to women. "
|
VIAGRA
(sildenafil citrate)
The Viagra story is interesting - in the context of this lecture -
because it emphasizes the importance of peripheral factors
in the control of sexual behaviour.
A pill hailed as a "magic bullet" for the way it combats male impotence
has created the greatest demand for a drug in the history of the
American pharmaceutical industry. Originally developed as a heart
medication, it is the first oral pill for impotence and it works for at
least 70 per cent of sufferers. There are side effects. For example,
about 16 per cent of men in the tests reported headaches. Another
potential problem is that the drugs may mask early warning signs of
heart disease.
The Virginia Urology Center explains Viagra's biochemical
mechanism as follows: "To achieve an erection the
brain produces a chemical (cyclic GMP) that relaxes specific muscles
and allows the blood to flow into the penis. After orgasm, another
chemical, (Phosphodiesterase) is produced to remove cyclic GMP, so
blood can flow out of the penis. Viagra™ is a
Phosphodiesterase Type (5 PDE-5) Inhibitor that, increases the time
cyclic GMP is available to achieve or maintain an erection for sexual
activities."
The underlying mechanisms of male and female impotence may be similar.
Female genitals fill with blood during sexual stimulation just as male
genitals do, resulting in engorgement of the clitoris and lubrication
of the vagina. Therefore female trials of Viagra are under way in
Europe and the U.S |

Figure 2.
Percentage of Patients Reporting an Improvement in Erections. |
The
following description of clinical studies of Viagra appeared on the Pfizer Viagra site
" The frequency of patients reporting improvement of
erections in response to a global question in four of the randomized,
double-blind, parallel, placebo controlled fixed dose studies (1797
patients) of 12 to 24 weeks duration is shown in Figure 2. These
patients had erectile dysfunction at baseline that was characterized by
median categorical scores of 2 (a few times) on principal IIEF
questions. Erectile dysfunction was attributed to organic (58%;
generally not characterized, but including diabetes and excluding
spinal cord injury), psychogenic (17%), or mixed (24%) etiologies.
Sixty-three percent, 74%, and 82% of the patients on 25 mg, 50 mg and
100 mg of VIAGRA, respectively, reported an improvement in their
erections, compared to 24% on placebo."
Further details can be obtained from the Pfizer Viagra site |
The
Virginia Urology Center Impotence/Sexual
Dysfunction site has detailed answers to the following
questions:
- How
does an erection occur?
- What
is impotence?
- What
can cause impotence?
- How
is impotence evaluated?
|
Copyright Dr.
C.A.P. Kenyon 1994-2006





 The estrus
cycle lasts four or five days in
the rat, and is associated with variations in the level of the hormones
estrogen
and progesterone released by the ovaries.
Around mid-cycle there is a 12-15
hour period of estrus or 'heat' during which the female is receptive
to the male.
Estrus is timed to occur when eggs are released from the ovaries
(ovulation) to increase
the likelihood that they will be fertilized by sperm from the male rat.
The estrus
cycle lasts four or five days in
the rat, and is associated with variations in the level of the hormones
estrogen
and progesterone released by the ovaries.
Around mid-cycle there is a 12-15
hour period of estrus or 'heat' during which the female is receptive
to the male.
Estrus is timed to occur when eggs are released from the ovaries
(ovulation) to increase
the likelihood that they will be fertilized by sperm from the male rat. Why do you think - at
one time - female rats were viewed as passive recipients of male sexual
sexual behaviour?
Why do you think - at
one time - female rats were viewed as passive recipients of male sexual
sexual behaviour? In the
first part of this lecture we
will examine the role of estrogen and progesterone in the sexual
behaviours of female
rats. This diagram shows that estrogen and progesterone levels increase
before ovulation,
and are at a relatively high level during estrus. A number of
experiments have examined
the roles of estrogen and progesterone on the sexual behaviours of
female rats.
In the
first part of this lecture we
will examine the role of estrogen and progesterone in the sexual
behaviours of female
rats. This diagram shows that estrogen and progesterone levels increase
before ovulation,
and are at a relatively high level during estrus. A number of
experiments have examined
the roles of estrogen and progesterone on the sexual behaviours of
female rats.

 Effects of progesterone
on sexual
behaviour in female rats
Effects of progesterone
on sexual
behaviour in female rats This diagram shows a
piece of
apparatus that can be used to measure female sexual motivation.
A male and a female
rat are enclosed in individual boxes with gauze windows placed within a
larger test
apparatus.
This diagram shows a
piece of
apparatus that can be used to measure female sexual motivation.
A male and a female
rat are enclosed in individual boxes with gauze windows placed within a
larger test
apparatus. 
 How might
estrogen affect sexual behaviour? It's
tempting to conclude from the results above showing the effect of
estrogen on sexual
receptivity that estrogen directly stimulates brain cells that control
behaviour.
How might
estrogen affect sexual behaviour? It's
tempting to conclude from the results above showing the effect of
estrogen on sexual
receptivity that estrogen directly stimulates brain cells that control
behaviour.  There are specific receptor sites in the brain
that act as target cells for
hormones released by the testes and ovaries. These are shown as red
blobs in the diagram.
There are specific receptor sites in the brain
that act as target cells for
hormones released by the testes and ovaries. These are shown as red
blobs in the diagram.  Sexual
behaviour in male rats consists of three
behaviours:
Sexual
behaviour in male rats consists of three
behaviours:
 Under
normal conditions male rats typically emit an intromission
every 20 to 30 seconds, and ejaculate after 10-12 intromissions.
Under
normal conditions male rats typically emit an intromission
every 20 to 30 seconds, and ejaculate after 10-12 intromissions. 

 In the
experiment shown here, carried
out on male guinea pigs (Grunt & Young, 1952), sexual behaviour
decreased shortly
after castration (gonadectomy) and was re-established by the
administration of
testosterone to castrated animals.
In the
experiment shown here, carried
out on male guinea pigs (Grunt & Young, 1952), sexual behaviour
decreased shortly
after castration (gonadectomy) and was re-established by the
administration of
testosterone to castrated animals.
 It is
tempting to think that the restoration of
sexual behaviour in castrated male animals given testosterone
replacement therapy is the
result of the hormone reactivating 'centers' in the
brain that control sexual
motivation.
It is
tempting to think that the restoration of
sexual behaviour in castrated male animals given testosterone
replacement therapy is the
result of the hormone reactivating 'centers' in the
brain that control sexual
motivation.  If you are
still not convinced by this argument that
recovery of sexual behaviour with testosterone replacement is the
result of recovery of
peripheral function, consider this diagram which shows the effect of
castration and
testosterone replacement therapy upon the number of genital papillae
and the number of
animals continuing to copulate four weeks after castration.
If you are
still not convinced by this argument that
recovery of sexual behaviour with testosterone replacement is the
result of recovery of
peripheral function, consider this diagram which shows the effect of
castration and
testosterone replacement therapy upon the number of genital papillae
and the number of
animals continuing to copulate four weeks after castration. 